Mole Fraction
In chemistry, the mole fraction, xi, is defined as the amount of moles of a constituent, ni, divided by the total amount of moles of all constituents in a mixture, ntot:
Mole fractions are dimensionless, and the sum of all mole fractions in a given mixture is always equal to 1.
Properties of the Mole Fraction
The mole fraction is used very frequently in the construction of phase diagrams. It has a number of advantages:
- It is not temperature dependent, as opposed to molar concentration, and does not require knowledge of the densities of the phase(s) involved.
- A mixture of known mole fractions can be prepared by weighing the appropriate masses of the constituents.
- The measure is symmetric; in the mole fractions x=0.1 and x=0.9, the roles of 'solvent' and 'solute' are reversible.
- In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to total pressure of the mixture.
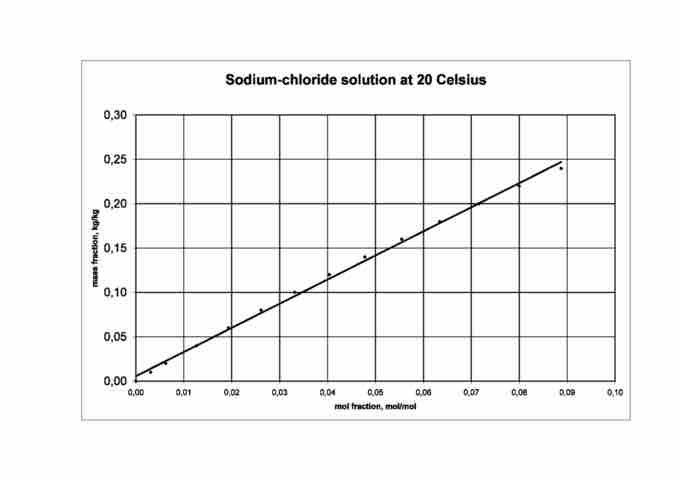
Mole fraction in a sodium chloride solution
Mole fraction increases proportionally to mass fraction in a solution of sodium chloride.
Mole Percent
Multiplying the mole fraction by 100 gives the mole percentage, also referred as amount/amount percent (abbreviated as n/n%). For general chemistry, all the mole percents of a mixture add up to 100 mole percent. We can easily convert mole percent back to mole fraction by dividing by 100. So, a mole fraction of 0.60 is equal to a mole percent of 60.0%.
Calculations with Mole fraction and Mole Percent
Mole Fraction in Mixtures
A mixture of gases was formed by combining 6.3 moles of O2 and 5.6 moles of N2. What is the mole fraction of nitrogen in the mixture?
First, we must find the total number of moles with ntotal = nN2 + nO2.
Next we must divide the moles of N2 by the total number of moles:
The mole fraction of nitrogen in the mixture is 0.47.
Mole Fraction in Solutions
Mole fraction can also be applied in the case of solutions. For example, 0.100 moles of NaCl are dissolved in 100.0 mL of water. What is the mole fraction of NaCl?
We are given the number of moles of NaCl, but the volume of water. First, we convert this volume to a mass by using the density of water (1.00 g/mL), and then we convert this mass to moles of water:
With this information we can find the total number of moles present: 5.55 + 0.100 = 5.65 moles. If we divide the moles of NaCl by the total number of moles, we find the mole fraction of this component:
We find that the mole fraction of NaCl is 0.0176.
Mole Fraction with Multi-Component Mixtures
Mole fractions can also be found for mixtures that are formed from multiple components. These are treated no differently than before; again, the total mole fraction of the mixture must be equal to 1.
For example, a solution is formed by mixing 10.0 g of pentane (C5H12), 10.0 g of hexane (C6H14) and 10.0 g of benzene (C6H6). What is the mole fraction of hexane in this mixture?
We must first find the number of moles present in 10.0 g of each component, given their chemical formulas and molecular weights. The number of moles for each is found by dividing its mass by its respective molecular weight. We find that there are 0.138 moles of pentane, 0.116 moles of hexane, and 0.128 moles of benzene.
We can find the total number of moles by taking the sum of all the moles: 0.138+0.116+0.128 = 0.382 total moles. If we divide moles of hexane by the total moles, we calculate the mole fraction:
The mole fraction for hexane is 0.303.
Mole Fraction from Molality
Mole fraction can also be calculated from molality. If we have a 1.62 m solution of table sugar (C6H12O6) in water, what is the mole fraction of the table sugar?
Since we are given molality, we can convert it to the equivalent mole fraction, which is already a mass ratio; remember that molality = moles solute/kg solvent. Given the definition of molality, we know that we have a solution with 1.62 moles of sugar and 1.00 kg (1000 g) of water. Since we know the number of moles of sugar, we need to find the moles of water using its molecular weight:
The total number of moles is the sum of the moles of water and sugar, or 57.1 moles total of solution. We can now find the mole fraction of the sugar:
With the mole fraction of 0.0284, we see that we have a 2.84% solution of sugar in water.
Mole Fraction from Mass Percent
The mole fraction can also be calculated from a mass percent. What is the mole fraction of cinnamic acid that has a mass percent of 50.00% urea in cinnamic acid? The molecular weight of urea is 60.16 g/mol and the molecular weight of cinnamic acid is 148.16 g/mol.
First, we assume a total mass of 100.0 g, although any mass could be assumed. This means that we have 50.0 g of urea and 50.0 g of cinnamic acid. We can then calculate the moles present by dividing each by its molecular weight. We have 0.833 moles urea and 0.388 moles cinnamic acid, so we have 1.22 moles total.
To find the mole fraction, we divide the moles of cinnamic acid by total number of moles:
The mole fraction for cinnamic acid is 0.318.