To ensure that you understand the material in this chapter, you should review the meanings of the bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
Lipids, found in the body tissues of all organisms, are compounds that are more soluble in organic solvents than in water. Many of them contain fatty acids, which are carboxylic acids that generally contain an even number of 4–20 carbon atoms in an unbranched chain. Saturated fatty acids have no carbon-to-carbon double bonds. Monounsaturated fatty acids have a single carbon-to-carbon double bond, while polyunsaturated fatty acids have more than one carbon-to-carbon double bond. Linoleic and linolenic acid are known as essential fatty acids because the human body cannot synthesize these polyunsaturated fatty acids. The lipids known as fats and oils are triacylglycerols, more commonly called triglycerides—esters composed of three fatty acids joined to the trihydroxy alcohol glycerol. Fats are triglycerides that are solid at room temperature, and oils are triglycerides that are liquid at room temperature. Fats are found mainly in animals, and oils found mainly in plants. Saturated triglycerides are those containing a higher proportion of saturated fatty acid chains (fewer carbon-to-carbon double bonds); unsaturated triglycerides contain a higher proportion of unsaturated fatty acid chains.
Saponification is the hydrolysis of a triglyceride in a basic solution to form glycerol and three carboxylate anions or soap molecules. Other important reactions are the hydrogenation and oxidation of double bonds in unsaturated fats and oils.
Phospholipids are lipids containing phosphorus. In phosphoglycerides, the phosphorus is joined to an amino alcohol unit. Some phosphoglycerides, like lecithins, are used to stabilize an emulsion—a dispersion of two liquids that do not normally mix, such as oil and water. Sphingolipids are lipids for which the precursor is the amino alcohol sphingosine, rather than glycerol. A glycolipid has a sugar substituted at one of the OH groups of either glycerol or sphingosine. All are highly polar lipids found in cell membranes.
Polar lipids have dual characteristics: one part of the molecule is ionic and dissolves in water; the rest has a hydrocarbon structure and dissolves in nonpolar substances. Often, the ionic part is referred to as hydrophilic (literally, “water loving”) and the nonpolar part as hydrophobic (“water fearing”). When placed in water, polar lipids disperse into any one of three arrangements: micelles, monolayers, and bilayers. Micelles are aggregations of molecules in which the hydrocarbon tails of the lipids, being hydrophobic, are directed inward (away from the surrounding water), and the hydrophilic heads that are directed outward into the water. Bilayers are double layers arranged so that the hydrophobic tails are sandwiched between the two layers of hydrophilic heads, which remain in contact with the water.
Every living cell is enclosed by a cell membrane composed of a lipid bilayer. In animal cells, the bilayer consists mainly of phospholipids, glycolipids, and the steroid cholesterol. Embedded in the bilayer are integral proteins, and peripheral proteins are loosely associated with the surface of the bilayer. Everything between the cell membrane and the membrane of the cell nucleus is called the cytoplasm.
Most lipids can be saponified, but some, such as steroids, cannot be saponified. The steroid cholesterol is found in animal cells but never in plant cells. It is a main component of all cell membranes and a precursor for hormones, vitamin D, and bile salts. Bile salts are the most important constituents of bile, which is a yellowish-green liquid secreted by the gallbladder into the small intestine and is needed for the proper digestion of lipids.
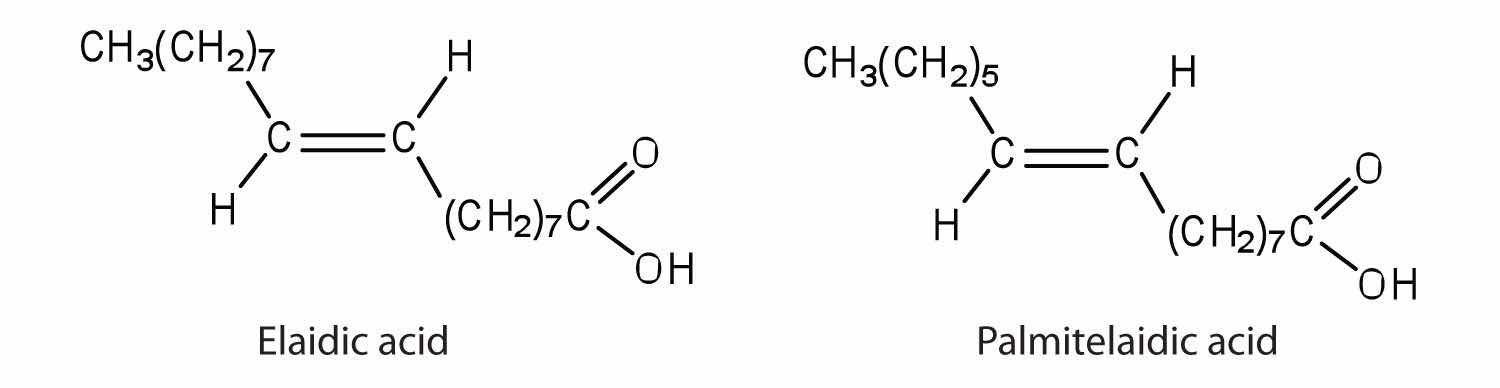
The melting point of elaidic acid is 52°C.
Would you expect the melting point of palmitelaidic acid to be lower or higher than that of elaidic acid? Explain.
Examine the labels on two brands of margarine and two brands of shortening and list the oils used in the various brands.
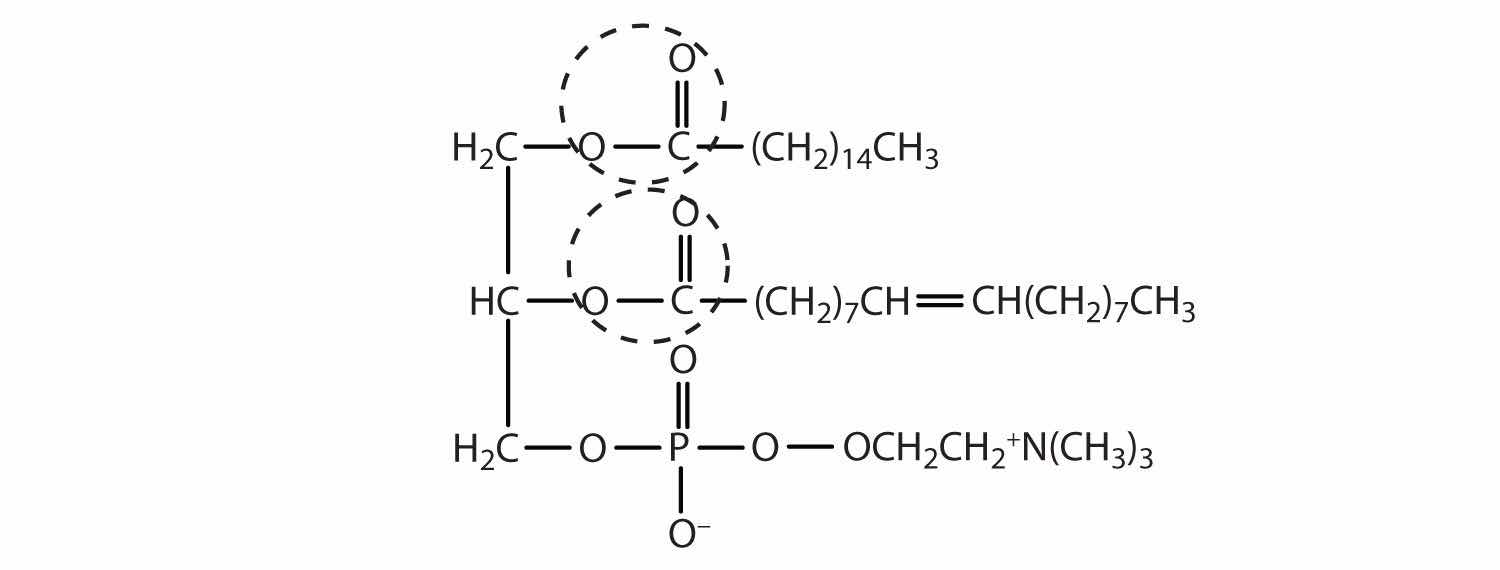
Draw a typical lecithin molecule that incorporates glycerol, palmitic acid, oleic acid, phosphoric acid, and choline. Circle all the ester bonds.
In cerebrosides, is the linkage between the fatty acid and sphingosine an amide bond or an ester bond? Justify your answer.
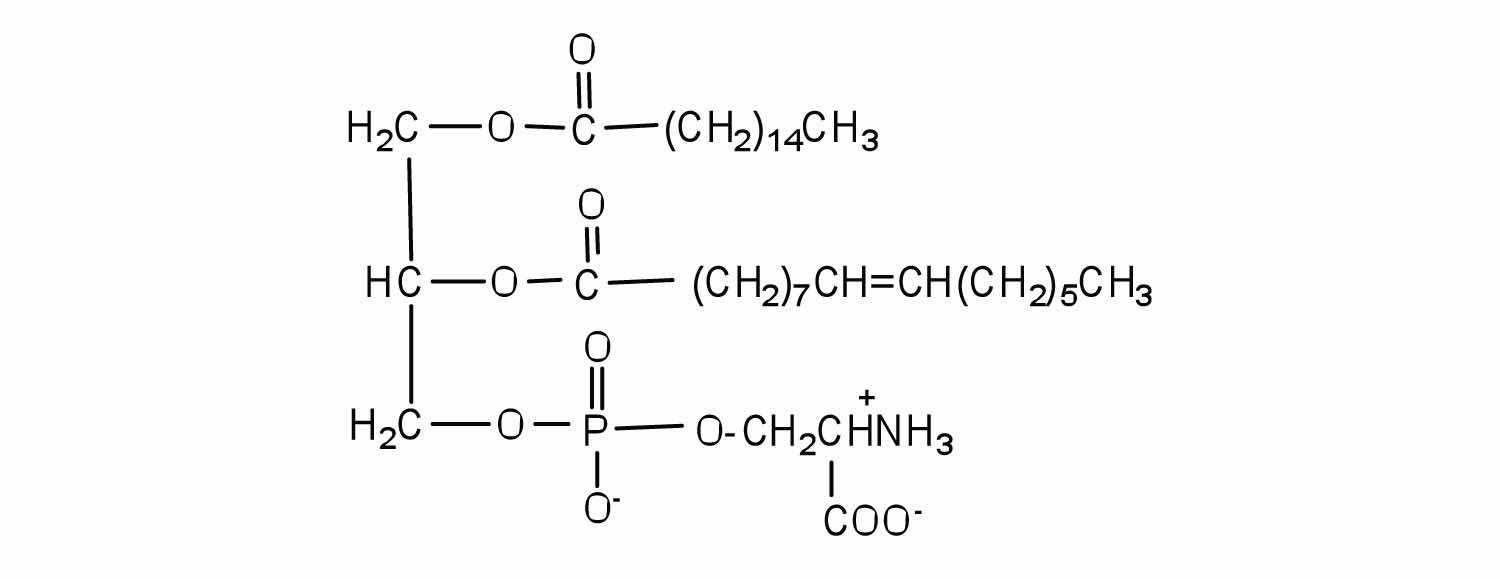
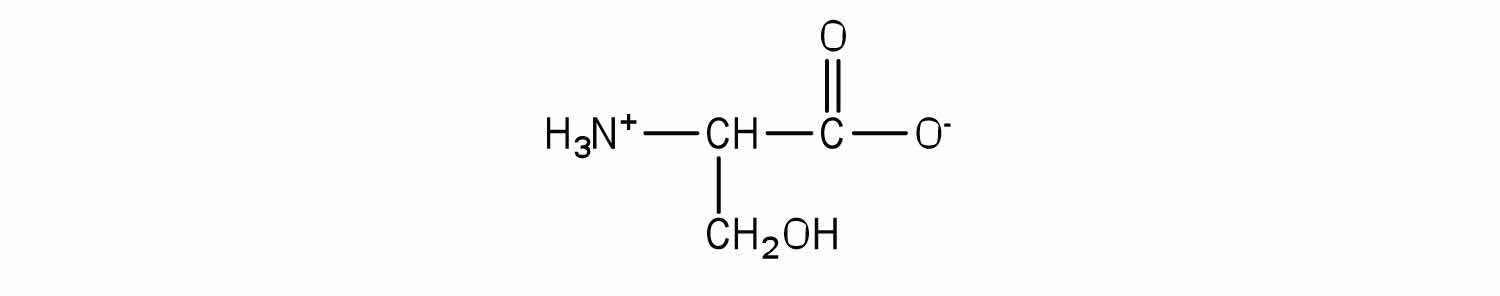
Serine is an amino acid that has the following structure. Draw the structure for a phosphatidylserine that contains a palmitic acid and a palmitoleic acid unit.
Explain whether each compound would be expected to diffuse through the lipid bilayer of a cell membrane.
Identify the role of each steroid hormone in the body.
How does the structure of cholic acid differ from that of cholesterol? Which compound would you expect to be more polar? Why?
Why is it important to determine the ratio of LDLs to HDLs, rather than just the concentration of serum cholesterol?