Monosaccharides such as glucose and fructose are crystalline solids at room temperature, but they are quite soluble in water, each molecule having several OH groups that readily engage in hydrogen bonding. The chemical behavior of these monosaccharides is likewise determined by their functional groups.
An important reaction of monosaccharides is the oxidation of the aldehyde group, one of the most easily oxidized organic functional groups. Aldehyde oxidation can be accomplished with any mild oxidizing agent, such as Tollens’ reagent or Benedict’s reagent. (For more information about aldehyde oxidation, see Chapter 14 "Organic Compounds of Oxygen", Section 14.5 "Reactions of Alcohols".) With the latter, complexed copper(II) ions are reduced to copper(I) ions that form a brick-red precipitate [copper(I) oxide; Figure 16.7 "Benedict’s Test"].
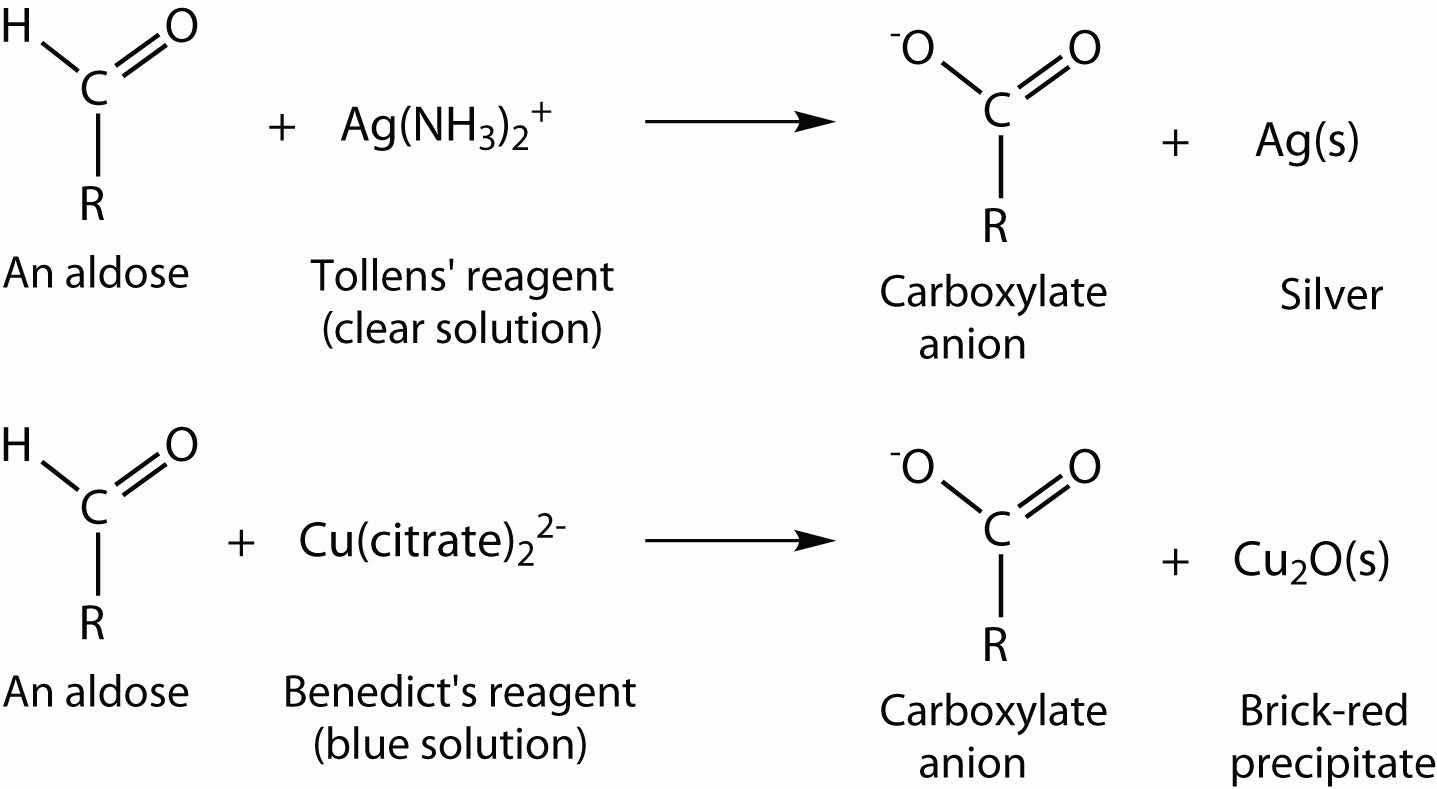
Any carbohydrate capable of reducing either Tollens’ or Benedict’s reagents without first undergoing hydrolysis is said to be a reducing sugarAny carbohydrate capable of reducing a mild oxidizing agent, such as Tollens’ or Benedict’s reagents, without first undergoing hydrolysis.. Because both the Tollens’ and Benedict’s reagents are basic solutions, ketoses (such as fructose) also give positive tests due to an equilibrium that exists between ketoses and aldoses in a reaction known as tautomerism.
Figure 16.7 Benedict’s Test

Benedict’s test was performed on three carbohydrates, depicted from left to right: fructose, glucose, and sucrose. The solution containing sucrose remains blue because sucrose is a nonreducing sugar.
These reactions have been used as simple and rapid diagnostic tests for the presence of glucose in blood or urine. For example, Clinitest tablets, which are used to test for sugar in the urine, contain copper(II) ions and are based on Benedict’s test. A green color indicates very little sugar, whereas a brick-red color indicates sugar in excess of 2 g/100 mL of urine.
Why are monosaccharides soluble in water?
What is a reducing sugar?
Monosaccharides are quite soluble in water because of the numerous OH groups that readily engage in hydrogen bonding with water.
any carbohydrate capable of reducing a mild oxidizing agent, such as Tollens’ or Benedict’s reagents, without first undergoing hydrolysis
Which gives a positive Benedict’s test—L-galactose, levulose, or D-glucose?
Which gives a positive Benedict’s test—D-glyceraldehyde, corn sugar, or L-fructose?
D-Galactose can be oxidized at the sixth carbon atom to yield D-galacturonic acid and at both the first and sixth carbon atoms to yield D-galactaric acid. Draw the Fischer projection for each oxidation product.
D-Glucose can be oxidized at the first carbon atom to form D-gluconic acid, at the sixth carbon atom to yield D-glucuronic acid, and at both the first and sixth carbon atoms to yield D-glucaric acid. Draw the Fischer projection for each oxidation product.
All three will give a positive Benedict’s test because they are all monosaccharides.
