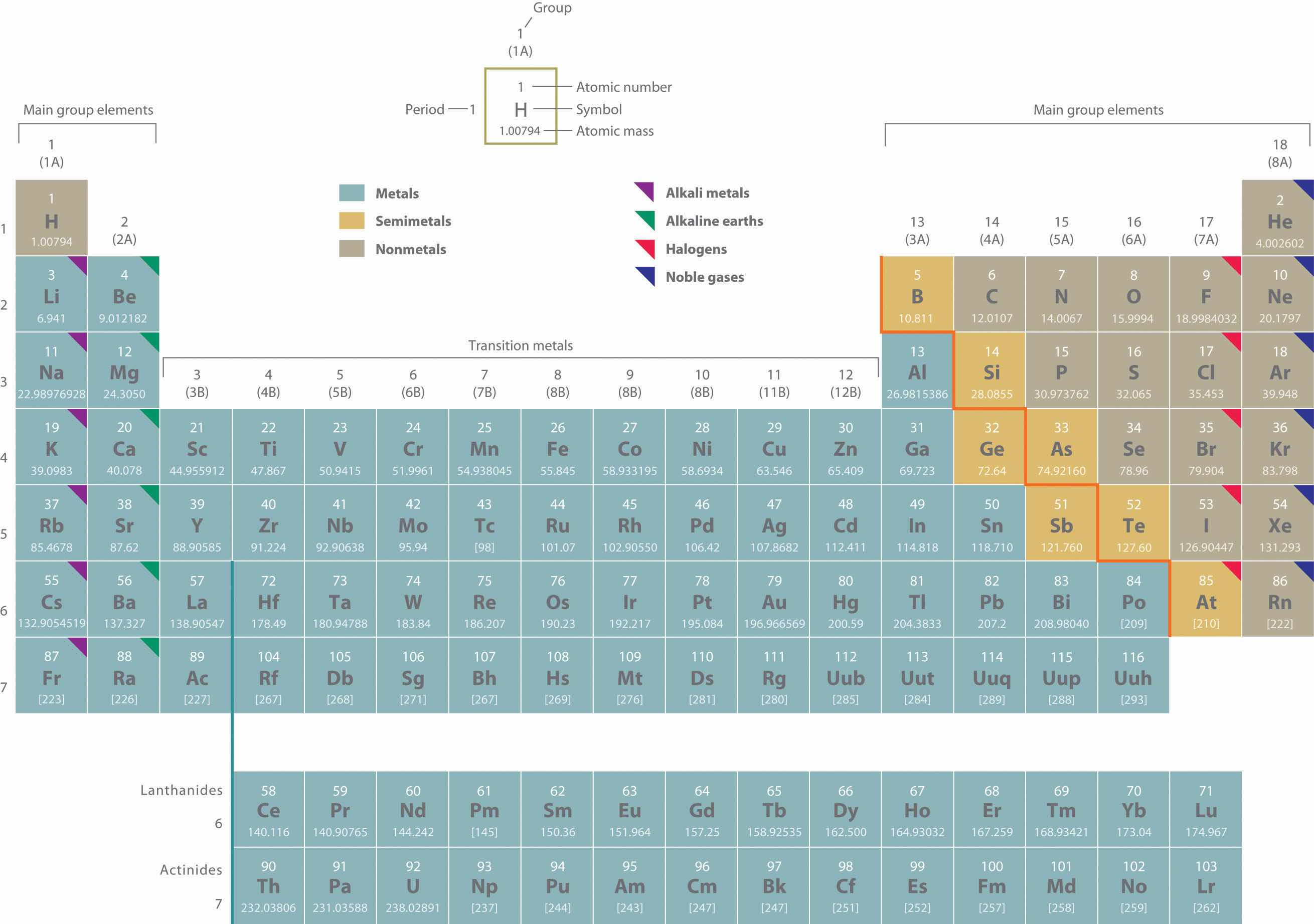
Two systems for numbering periodic groups are shown: 1–18 is the system currently recommended by the Inernational Union of Pure and Applied Chemistry (IUPAC); an older U.S. system, in which letters designate main group elements (A) and transition elements (B), is given parentheses.
An atomic mass in brackets indicates the mass of the longest-lived isotope of an element having no stable isotopes.
Elements with atomic numbers 114 (ununquadium, 289 amu) and 116 (ununhexium, 293 amu) have been recognized by the International Union of Pure and Applied Chemistry (IUPAC). The collaborating scientists from the Joint Institute for Nuclear Research in Dubna, Russia, and Lawrence Livermore National Laboratory in California have been invited to propose names for the new elements. See http://iupac.org/publications/pac/asap/PAC-REP-10-05-01
| List of Elements | |||
|---|---|---|---|
| Name | Symbol | Atomic Number | Atomic Mass |
| Actinium | Ac | 89 | [227]* |
| Aluminum | Al | 13 | 26.9815386(8) |
| Americium | Am | 95 | [243]* |
| Antimony | Sb | 51 | 121.760(1) |
| Argon | Ar | 18 | 39.948(1) |
| Arsenic | As | 33 | 74.92160(2) |
| Astatine | At | 85 | [210]* |
| Barium | Ba | 56 | 137.327(7) |
| Berkelium | Bk | 97 | [247]* |
| Beryllium | Be | 4 | 9.012182(3) |
| Bismuth | Bi | 83 | 208.98040(1) |
| Bohrium | Bh | 107 | [267]* |
| Boron | B | 5 | 10.811(7) |
| Bromine | Br | 35 | 79.904(1) |
| Cadmium | Cd | 48 | 112.411(8) |
| Calcium | Ca | 20 | 40.078(4) |
| Californium | Cf | 98 | [251]* |
| Carbon | C | 6 | 12.0107(8) |
| Cerium | Ce | 58 | 140.116(1) |
| Cesium | Cs | 55 | 132.9054519(2) |
| Chlorine | Cl | 17 | 35.453(2) |
| Chromium | Cr | 24 | 51.9961(6) |
| Cobalt | Co | 27 | 58.933195(5) |
| Copernicium† | Cn | 112 | [285]* |
| Copper | Cu | 29 | 63.546(3) |
| Curium | Cm | 96 | [247]* |
| Darmstadtium | Ds | 110 | [281]* |
| Dubnium | Db | 105 | [268]* |
| Dysprosium | Dy | 66 | 162.500(1) |
| Einsteinium | Es | 99 | [252]* |
| Erbium | Er | 68 | 167.259(3) |
| Europium | Eu | 63 | 151.964(1) |
| Fermium | Fm | 100 | [257]* |
| Fluorine | F | 9 | 18.9984032(5) |
| Francium | Fr | 87 | [223]* |
| Gadolinium | Gd | 64 | 157.25(3) |
| Gallium | Ga | 31 | 69.723(1) |
| Germanium | Ge | 32 | 72.64(1) |
| Gold | Au | 79 | 196.966569(4) |
| Hafnium | Hf | 72 | 178.49(2) |
| Hassium | Hs | 108 | [269]* |
| Helium | He | 2 | 4.002602(2) |
| Holmium | Ho | 67 | 164.93032(2) |
| Hydrogen | H | 1 | 1.00794(7) |
| Indium | In | 49 | 114.818(3) |
| Iodine | I | 53 | 126.90447(3) |
| Iridium | Ir | 77 | 192.217(3) |
| Iron | Fe | 26 | 55.845(2) |
| Krypton | Kr | 36 | 83.798(2) |
| Lanthanum | La | 57 | 138.90547(7) |
| Lawrencium | Lr | 103 | [262]* |
| Lead | Pb | 82 | 207.2(1) |
| Lithium | Li | 3 | 6.941(2) |
| Lutetium | Lu | 71 | 174.967(1) |
| Magnesium | Mg | 12 | 24.3050(6) |
| Manganese | Mn | 25 | 54.938045(5) |
| Meitnerium | Mt | 109 | [276]* |
| Mendelevium | Md | 101 | [258]* |
| Mercury | Hg | 80 | 200.59(2) |
| Molybdenum | Mo | 42 | 95.94(2) |
| Neodymium | Nd | 60 | 144.242(3) |
| Neon | Ne | 10 | 20.1797(6) |
| Neptunium | Np | 93 | [237]* |
| Nickel | Ni | 28 | 58.6934(2) |
| Niobium | Nb | 41 | 92.90638(2) |
| Nitrogen | N | 7 | 14.0067(2) |
| Nobelium | No | 102 | [259]* |
| Osmium | Os | 76 | 190.23(3) |
| Oxygen | O | 8 | 15.9994(3) |
| Palladium | Pd | 46 | 106.42(1) |
| Phosphorus | P | 15 | 30.973762(2) |
| Platinum | Pt | 78 | 195.084(9) |
| Plutonium | Pu | 94 | [244]* |
| Polonium | Po | 84 | [209]* |
| Potassium | K | 19 | 39.0983(1) |
| Praseodymium | Pr | 59 | 140.90765(2) |
| Promethium | Pm | 61 | [145]* |
| Protactinium | Pa | 91 | 231.03588(2)* |
| Radium | Ra | 88 | [226]* |
| Radon | Rn | 86 | [222]* |
| Rhenium | Re | 75 | 186.207(1) |
| Rhodium | Rh | 45 | 102.90550(2) |
| Roentgenium | Rg | 111 | [280]* |
| Rubidium | Rb | 37 | 85.4678(3) |
| Ruthenium | Ru | 44 | 101.07(2) |
| Rutherfordium | Rf | 104 | [267]* |
| Samarium | Sm | 62 | 150.36(2) |
| Scandium | Sc | 21 | 44.955912(6) |
| Seaborgium | Sg | 106 | [271]* |
| Selenium | Se | 34 | 78.96(3) |
| Silicon | Si | 14 | 28.0855(3) |
| Silver | Ag | 47 | 107.8682(2) |
| Sodium | Na | 11 | 22.98976928(2) |
| Strontium | Sr | 38 | 87.62(1) |
| Sulfur | S | 16 | 32.065(5) |
| Tantalum | Ta | 73 | 180.94788(2) |
| Technetium | Tc | 43 | [98]* |
| Tellurium | Te | 52 | 127.60(3) |
| Terbium | Tb | 65 | 158.92535(2) |
| Thallium | Tl | 81 | 204.3833(2) |
| Thorium | Th | 90 | 232.03806(2)* |
| Thulium | Tm | 69 | 168.93421(2) |
| Tin | Sn | 50 | 118.710(7) |
| Titanium | Ti | 22 | 47.867(1) |
| Tungsten | W | 74 | 183.84(1) |
| Ununhexium | Uuh | 116 | [293]* |
| Ununpentium | Uup | 115 | [288]* |
| Ununquadium | Uuq | 114 | [289]* |
| Ununtrium | Uut | 113 | [284]* |
| Uranium | U | 92 | 238.02891(3)* |
| Vanadium | V | 23 | 50.9415(1) |
| Xenon | Xe | 54 | 131.293(6) |
| Ytterbium | Yb | 70 | 173.04(3) |
| Yttrium | Y | 39 | 88.90585(2) |
| Zinc | Zn | 30 | 65.409(4) |
| Zirconium | Zr | 40 | 91.224(2) |
| *Element has no stable isotope. A value enclosed in brackets, e.g. [209], indicates the mass number of the longest-lived isotope of the element. Three such elements (Th, Pa, and U), however, do have a characteristic terrestrial isotopic composition, and an atomic mass is given for them. An uncertainty in the last digit in the Atomic Mass column is shown by the number in parentheses; e.g., 1.00794(7) indicates ±0.00007. | |||
| †Element 112 named shortly before the release of this text. Other periodic tables in this version of the text may refer to it as Ununbium (Uub). | |||
Source of data: Atomic weights of the elements 2001 (IUPAC Technical Report) as supplemented by the Table of Standard Atomic Weights 2005 (to be published in Pure and Applied Chemistry) on the IUPAC web site, and “Nuclear Data Sheets for A-266-294” (to be published in Nuclear Data Sheets) at http://www.nndc.bnl.gov/superheavy.pdf.