After exercise, the concentration of lactic acid increases in both muscle tissue and blood. In fact, it is responsible for muscle cramps that may develop after vigorous exercise. Using the structure of lactic acid shown here, draw the conformational isomers of lactic acid as viewed along the C2–C3 axis, where C3 is the carbon of the methyl group. Which would you predict to be the most stable conformation? Why?

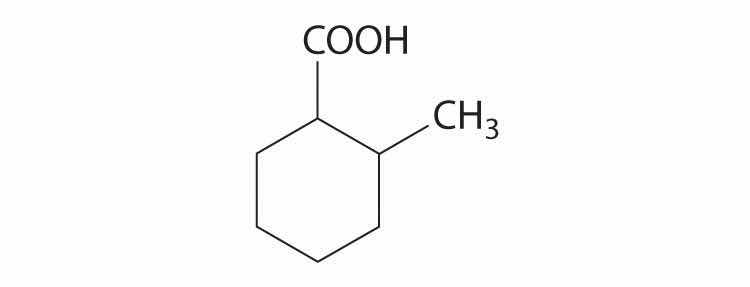
Cyclohexanecarboxylic acid is a liquid used in insecticide formulations. A derivative of this compound, 2-methylcyclohexanecarboxylic acid is shown here. Sketch the cis and trans isomers of this derivative. Arrange the conformations of its geometric isomers in order of increasing energy.
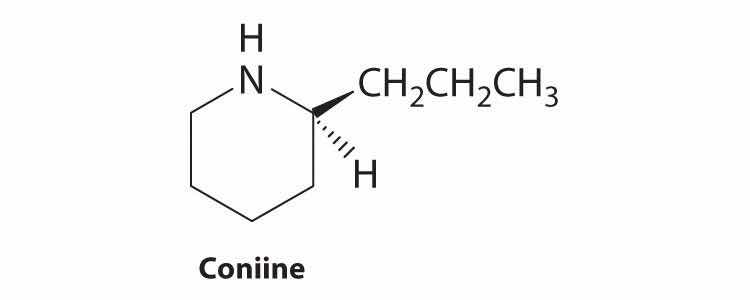
Coniine is a naturally occurring compound with insect-paralyzing properties. Ingestion of this compound causes weakness, vomiting, labored respiration, and eventual death. Does coniine have a chiral carbon? If so, indicate the carbon with an asterisk.
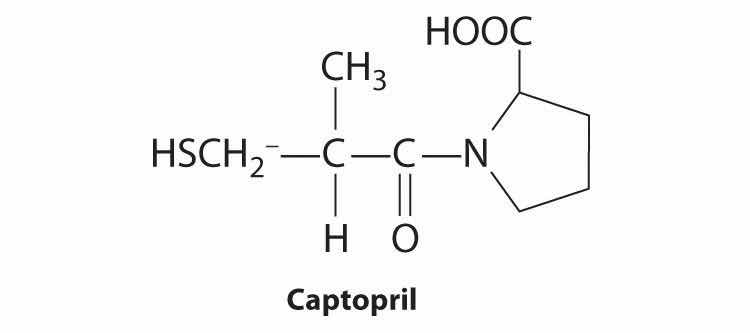
A compound that has been found to be an effective hypertensive is captopril, sold commercially as Hypertil and Tensoprel, among other names. Captopril has a chiral center at C2. Draw the enantiomers that result from this chiral center. Indicate any other chiral centers in captopril with an asterisk.
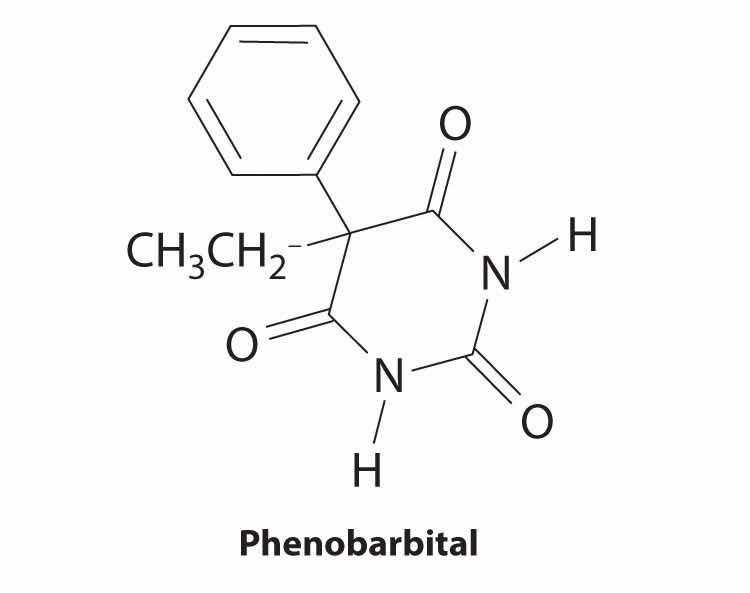
The structure of phenobarbital, a compound used to treat epilepsy, is shown here. Identify the functional groups.
The compound 2-amino-2-methyl-1-propanol is used not only in the synthesis of pharmaceuticals but also in cosmetic creams, polishes, and cleaning compounds. Is it a chiral compound?
Glycerol (1,2,3-propanetriol, or 1,2,3-trihydroxypropane) is produced from sugars by fermentation. It also is obtained from oils and fats as a by-product during the manufacture of soaps. Glycerol can be converted to glyceric acid by the following sequence:
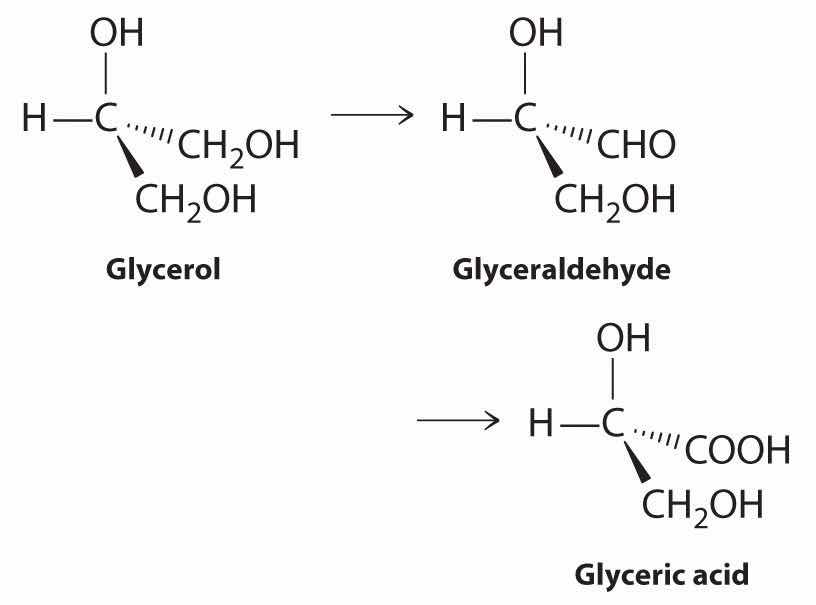
Both glyceraldehyde and glyceric acid are derivatives of biochemical intermediates in sugar metabolism.
When exposed to light or heat, peroxides (ROOR) can undergo radical reactions. The first step is the dissociation of the peroxide into two alkoxy radicals. Each alkoxy radical then reacts with a species such as HBr to form an alcohol.
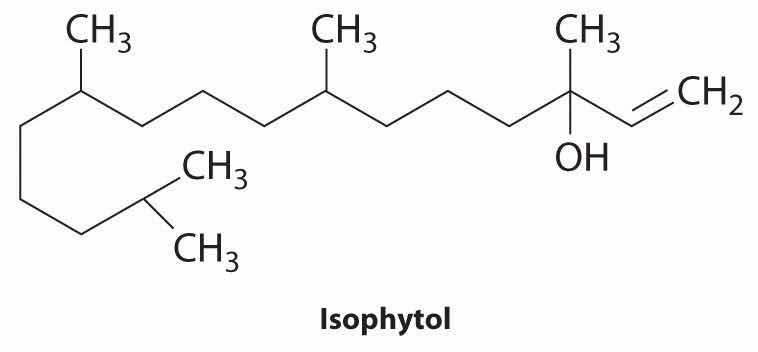
The structure of isophytol is shown here. This compound is used to prepare vitamins E and K. Does isophytol form cis and trans isomers? If so, draw these isomers.
Isopentyl acetate, also known as “oil of banana,” has the structure shown here. Show the first step in the mechanism for reaction of isopentyl acetate with C6H5MgBr, which gives the first ionic adduct.

An enkephalin is a pentapeptide that controls pain in humans. Enkephalins function by binding to the same specific sites in brain cells that are known to bind morphine and heroin. Methionine enkephalin has the structure Tyr-Gly-Gly-Phe-Met.
The first staggered conformation minimizes electrostatic repulsions between adjacent atoms:

yes

three amides and a phenyl group