Downy mildew
 |
Downy mildew
Scientific name:
Albugo spp., Bremia spp., Peronospora spp., Pseudoperonospora spp.,
Family:
Peronosporales: Peronosporaceae
Type:
disease (fungal)
Host plants: Cabbage/Kale, Brassicas
Cucumber
Millet
Onion
Peas
Pumpkin
Soybean
Spinach
Watermelon
Zucchini/Courgette
Grapes, hops
|
Geographical distribution
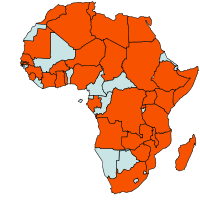 |
| Geographical Distribution of Downy mildew in Africa (red marked) |
|
Damage
Damage caused by downy mildews is usually associated with the sporulation of the fungus. Sporulation of Peronospora destructor can cause up to 55% reduction in the dry weight of onion leaves (Yarwood, 1941). The corresponding figures for Pseudoperonospora humuli on hops and Peronospora farinose on spinach were 17 and 48%, respectively. Losses from downy mildews can be considerable. It is estimated that in 1962 downy mildew of tobacco (Perenospora tabacina) reduced yields in Europe by at least 100,000 metric tons (Peyrot, 1962). Symptoms
Plants can be infected at any time during their growing period. Symptoms of downy mildew infection include small, pale yellow spots with indefinite borders on the upper leaf surface. Purplish discolouration of the upper leaf surface is seen on some hosts. A downy growth (sporangiophores) may be seen directly under the spots on the underside of the leaf or on fruits or stems early in the morning or when foliage is wet. Young leaves and cotyledons may drop off when yellow. Thus, the disease can cause severe damage to seedlings in the seedbed. Older leaves usually remain attached, and affected areas enlarge, turning brown and papery. When the disease is severe, whole leaves die. |
|
Affected plant stages
Seedling stage, vegetative growing stage, flowering stage and fruiting stage. Affected plant parts
Leaves and whole plant. Symptoms on affected plant part
Leaves: lesions; fungal growth.Stems: fungal growth.
Flowers: fungal growth; flower abortion; flower drop.
Fruiting stage: fungal growth.
Downy mildew fungi are fairly host specific. The downy mildew fungus that infects one type of plant (e.g., rose) is not the same downy mildew fungus that infects another (e.g., grape). However, if you see downy mildew on one plant, then environmental conditions (i.e., cool, wet weather) are favourable for development of downy mildews on a wide range of plants.
Downy mildew of grape, spinach, and tobacco causes serious economic losses. It spreads rapidly through fields and is dependent on a wet, humid environment with cool or warm, but not hot, temperatures. A film of water is needed on plant tissue for spore germination and infection.
Conditions that favour development include:
- Cool, moist weather conditions
- Host weeds found in between the crops
- Crop residues in the field
- Poor plant aeration
- Overcrowding (planting in high densities)
Pest and disease Management: General illustration of the concept of infonet-biovision
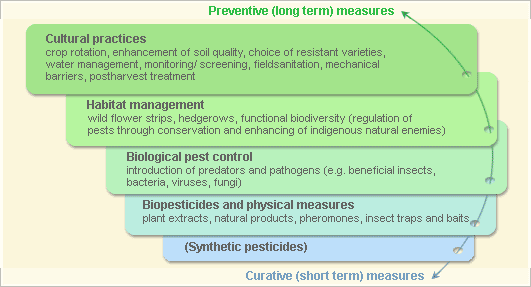
Further below you find concrete preventive and curative methods against Downy mildew.
Prevention
1. Use resistant varieties where available 2. Use only certified diseased-free seeds for sowing. Transplant only healthy seedlings.
3. Ensure proper land preparation to make sure that your soil is well drained.
4. Provide adequate plant spacing to reduce the density of the canopy and minimise humidity.
5. Pruning of new growth also helps proper plant's aeration.
6. Remove infected plants and prune infected shoots.
7. Properly dispose of collected diseased-parts either by burning or burying them.
8. Avoid overhead watering. It lengthens the duration of leaf wetness and favours further development of the disease.
9. Plough-under all the plant debris after harvest.
10. Practice crop rotation.
Control options
Control should be emphasised in nurseries since downy mildew is particularly damaging in the seedbed. 1. Seedbeds should have well-drained soils and be sited away from hedges and windbreaks. The site should not have been under susceptible crops for at least the previous 2 years.
2. Seedlings should not be excessively watered.
3. Weeds should be eradicated in and near seedbeds and out in the production fields.
4. Crop residues should be removed from the field after harvest.
5. Avoid sprinkler irrigation.
6. Thin plants to reduce plant density and increase air movement.
7. Time irrigations so that they do not elongate leaf wetness.
8. Alter planting dates to avoid periods of high disease pressure.
Copper
Garlic bulb extract
- Dobson, H., Cooper, J., Manyangarirwa, W., Karuma, J. and Chiimba, W. (2002). Integrated Vegetable Pest Management. Natural Resources Institute (UK). ISBN: 0-85954-536-9.
- ICIPE (2003).Varela, A.M., Seif, A.A. and Löhr, B. (2003). A Guide to IPM in Brassicas Production in Eastern and Southern Africa. ICIPE, Kenya. ISBN: 92 9064 148 7 www.icipe.org
- OISAT: Organisation for Non-Chemical Pest Management in the Tropics www.oisat.org
- Olis, J. and Hudelson, B. (2001). Downy mildew. BS thesis in Plant Pathology at the University of Wisconsin Madison. UW-Madison Plant Pathology. http://hort.uwex.edu
- Peyrot, J. (1962). Tobacco blue mould in Europe. FAO Plant Protection Bulletin 10, 73-80
- Wheeler, B.E.J. (1972). An Introduction to Plant Diseases. The English Language Book Society and John Wiley & Sons Ltd. ISBN: 0 471 03752 5
- Yarwood, C. E. (1941). Sporulation injury associated with downy mildew infections. Phytopathology, 31, 741-748
