Black rot
 |
Black rot
Scientific name:
Xanthomonas campestris pv. campestris
Family:
Xanthomonadales: Xanthomonadaceae
Type:
disease (bacterial)
Host plants: Cabbage/Kale, Brassicas
Pumpkin
Sweet potato
Mustard, Radish, Crucifers
|
Geographical distribution
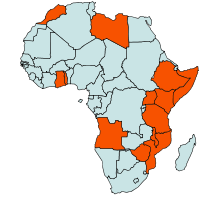 |
| Geographical Distribution of Black rot in Africa (red marked) |
Introduction
Black rot affects cabbage and related crops (brassicas, mustard & radish) worldwide and is caused by the bacterium Xanthomonas campestris pv. campestris. Black rot is one of the most serious cabbage / kale diseases in warm climates. Diseased plants may rot quickly before or after harvest because of secondary infection from bacterial soft-rot. Soft-rot bacteria may invade heads of black-rot-infected plants, causing tissue to become slimy and foul-smelling. The black rot bacterium can over-season on infected cabbage seeds, in weeds belonging to the Brassica family (including: black mustard, field mustard, wild turnip, wild radish, shepherd's purse, and pepper weed); or in infected plant material in the soil. The bacterium can persist in plant residue for 1-2 years or as long as the plant debris remains intact.
Damage
In Kenya, black rot is endemic and the cause of much damage (Onsando, 1988, 1992). The disease is considered of intermediate economic importance in Mozambique (Plumb-Dhindsa and Mondjane, 1984). Black rot is widespread in Zimbabwe where it is considered the most important disease of brassicas (Mguni, 1987, 1995). Host range
Black rot is a pathogen of most cultivated cruciferous plants and weeds. Cauliflower and cabbage are the most readily affected hosts in the crucifers, although kale is almost equally susceptible. Broccoli and Brussels sprouts have intermediate resistance and radish is quite resistant, but not to all strains. Kohlrabi, Chinese cabbage, rutabaga, turnip, collard, rape, jointed charlock (Raphanus raphanistrum) and mustard are also susceptible hosts. Symptoms on cabbage
The plant can be infected at any time during its life cycle. On young seedlings a yellowing appears along the margin of the cotyledons, which later shrivels and drops off. On the margins of mature leaves, similar yellowing appears. Initially, a small V-shaped area develops, but as the diseased area enlarges, the veins become distinctly black. In contrast to Fusarium yellows the veins are brownish in colour. The affected stem, when cut crosswise, shows a characteristic black ring. In later stages the entire head may turn black and soft due to secondary infection by soft rot bacteria (Erwinia carotovora var.carotovora).
|
|
Affected plant stages
Seedling stage, vegetative growing stage and heading stage (cabbages). Affected plant parts
Leaves, seeds, stems, vegetative organs and whole plant. Symptoms by affected plant part
Leaves: 'V' shaped lesionsSeeds: discolourations; lesions.
Stems: Internal discoloration (black in colour) .
Vegetative organs: internal discolouration (black in colour); dry rot.
Whole plant: plant death
Source of infection and spread
The bacteria survive in infected seed, in debris from diseased plants left in the field and in infested soil. Seed-borne bacteria can be disseminated long distances. Many cruciferous weeds can harbour the black-rot bacteria. In a new field, black rot is usually introduced via infected seed or diseased transplants. Further spread is facilitated by water-splash, running water, and handling infected plants. The bacteria enter the plant mainly through water pores at the edges of leaves. They can also enter through the root system and wounds made by chewing insects. They then move through the water vessels to the stem and the head. Black rot is favoured by warm (26-30°C) wet conditions.
Pest and disease Management: General illustration of the concept of infonet-biovision
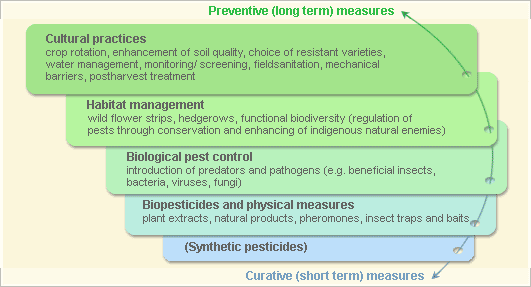
Further below you find concrete preventive and curative methods against Black rot.
Control options
- Use certified disease-free seed.
- Establish crops in seedbeds in black rot-free soils that have not grown crops from the family Crucifers for at least 3 years.
- Seedlings should not be crowded in the nursery.
- Transplants should not be dipped in water before transplanting.
- Mulching of the field crop, where practicable, is highly recommended.
- Avoid wet, poorly-drained soils
- Avoid overhead irrigation.
- Field operations during wet weather should be discouraged.
- Keep the field free of weeds, particularly of the crucifer family.
- Growing cabbage on raised beds helps eliminate conditions that induce black rot.
- When possible, remove, burn, or plough down all crop debris immediately after harvest to reduce the amount of bacteria in the soil
- A crop rotation based on at least a 2-year break in cruciferous crops is advocated.
- Use of resistant/tolerant varieties, where commercially available, provides the most effective control of the disease.
Hot water seed treatment
- Beije, C.M., Kanyangia, S.T., Muriuki, S.J.N., Otieno, E.A., Seif, A.A. and Whittle, A.M. (1984). Horticultural Crops Protection Handbook. National Horticultural Research Station, Thika, Kenya.
- CABI (2005). Crop Protection Compendium, 2005 Edition. © CAB International Publishing. Wallingford, UK. www.cabi.org
- Mguni C.M. (1987). Diseases of crops in the semiarid areas of Zimbabwe: can they be controlled economically. In: Cropping in the Semiarid Areas of Zimbabwe. Workshop Proceedings, Vol. 2 , Harare. Agritex, 417-433.
- Mguni C.M. (1995). Cabbage Research in Zimbabwe. Brassica Planning Workshop for East and South Africa Region. Lilongwe, Malawi, 15-18 May. GTZ/IPM Horticulture, Nairobi, Kenya, 31.
- Mguni C.M., 1996. Bacterial Black Rot (Xanthomonas campestris pv. campestris) of Vegetable Brassicas in Zimbabwe. PhD thesis, Department of Plant Biology. The Royal Veterinary and Agricultural University, Copenhagen and Danish Government Institute for Developing Countries, Hellerup, Denmark
- Natural Resources Institute (UK) (2002). Integrated Vegetable Pest Management by Hans Dobson, Jerry Cooper, Walter Manyangarirwa, Joshua Karuma and Wilfred Chiimba. ISBN: 0-85954-536-9
- Nega, E., Ulrich, R., Werner, S. und Jahn, M. (2003). Hot water treatment of vegetable seed – an alternative seed treatment method to control seed borne pathogens in organic farming. Journal of Plant Diseases and Protection 110(3):pp. 220-234. orgprints.org
- Onsando JM, 1992. Black rot of crucifers. Plant diseases of international importance. Volume. II. Diseases of vegetables and oil seed crops., 243-252.
- Onsando, J.M. (1992). Black rot of crucifers. In: Chaube H. S., U. S. Singh, A. N. Mukhopadyay and J. Kumar (eds), Plant Diseases of International Importance. Diseases of Vegetables and Oil Seed Crops, pp. 243-252. Prentice Hall, Englewood Cliffs, NJ.
- Onsando, J.M. (1988). Management of black rot of cabbage caused by Xanthomonas campestris pv. campestris in Kenya. Acta Horticulturae, No. 218:311-314.
- Varela, A.M., Seif, A.A. and Löhr, B. (2003). A Guide to IPM in Brassicas Production in Eastern and Southern Africa. ICIPE, Nairobi, Kenya. ISBN: 92 9064 148 7

